Photon energy is the amount of energy carried by a single photon. It depends upon a photon's frequency or wavelength. And because photons in a vacuum always travel at the same speed (the speed of light), the two quantities are related. Frequency and wavelength, at least in the world of electromagnetic radiation, are inverses. The higher the frequency, the shorter the wavelength. And the longer the wavelength, the lower the frequency.
{eq}E = hf {/eq}
In the equation for photon energy listed above, E stands for "photon energy," h stands for Planck's constant, and f stands for "frequency." The higher a photon's frequency, the more energy that photon carries.
Photon Wavelength
Imagine a wave traveling across the surface of the ocean. On some days they are small ripples, on others, they are large and powerful. You could classify them according to their height or the amount of energy they carry. But you could also classify them according to how frequently they hit the shore. Or you could classify them according to their length or the distance between two peaks or two troughs. Of course, these last two classifications (frequency and wavelength) would be related.
When it comes to classifying photons of light, the wavelengths (and frequencies) are separated into seven distinct categories: radio waves, microwaves, infrared light, visible light, ultraviolet light, X-rays, and gamma rays. Radio waves have the lowest frequency and the longest wavelength. Gamma rays have the highest frequency and shortest wavelength.
 |
Visible light can be further separated into colors. The colors of the rainbow are, in fact, a ranking of the photons according to wavelength. Red has the longest wavelength and lowest frequency, followed by orange and yellow. Then comes green and blue. The photons with the shortest wavelength and highest frequency are purple. Colors are just the human brain's interpretation of the detection of various photons.
It's important to note that the concept of "visible light" is a decidedly human one. The human eye can detect only certain photons; we can't see infrared, for example. But many snakes can. And many birds can see ultraviolet light.
To unlock this lesson you must be a Study.com Member.
Create your account
Photons can be emitted in a variety of ways. Because photons are simply electromagnetic waves traveling through space, the creation of that wave is always the result of the motion of charged particles. Below are a few common examples.
- Blackbody Radiation: Because atoms are made up of particles with a charge (protons and electrons), and because atoms vibrate and move as a function of their temperature, all objects emit something called blackbody radiation. This is why hot coals glow at the bottom of a fire long after the fuel has been consumed. As the atoms move, the shape and energy of the electron orbitals change, emitting photons that are typically infrared, at least at everyday temperatures.
- Spontaneous Emission: When electrons in a particle fall from a higher, more excited orbital to a lower one, a photon can be spontaneously emitted. In a given atom, the dropping electrons will produce a very distinct frequency of light of a single color. This is how neon lights operate.
- Radioactive Decay: The decay of radioactive particles can emit very high-frequency, very dangerous photons called gamma rays. This emission typically occurs when an atom's nucleus falls into a more stable configuration. The nuclear waste left over from modern power plants can emit this type of radiation for thousands of years.
To unlock this lesson you must be a Study.com Member.
Create your account
Scientists have been working to further our understanding of light since ancient times. Plato himself thought that light was made of rays emitted by the eyes. And when the rays struck an object, they allowed us to interpret its color, size, and shape.
In 1027, the Arab scientist Alhazen published his book of optics, which proved that light originated from light sources. He also invented the first pinhole camera and used his theories to explain why the image in the camera was upside-down.
Later, scientists like Isaac Newton and Christiaan Huygens argued about whether light was a particle or a wave. Newton was in the particle camp, while Huygens argued for the wave camp. Hundreds of years later, James Clerk Maxwell published his equations. And in 1900, Max Planck theorized that the energy of light is proportional to its frequency. His work eventually Einstein's theory that light exists in discrete quanta of energy (photons). Einstein also discovered the "photoelectric effect", which helped us understand how incoming photons can interact with, and eventually eject, electrons from a material. The higher the frequency of the light being used, the more energy the ejected electrons will have. It's how modern solar panels can turn light energy into electricity.
Our modern understanding of light is built upon the contributions of each of these scientists and many others. We now understand that light is both a wave and a particle. Or, at the very least, light is its own special thing that has the properties of both.
To unlock this lesson you must be a Study.com Member.
Create your account
A photon is a tiny packet of light energy. Strangely, photons have properties of both particles and waves. They make up all of the light we can see and many kinds we cannot see as well. The equations of James Clerk Maxwell helped us understand this wave-particle duality.
The amount of energy carried by a single photon is called photon energy. It depends upon a photon's frequency or wavelength. These two quantities are related. The higher the frequency, the shorter the wavelength, and the longer the wavelength, the lower the frequency. Scientists typically divide light into seven categories based on the frequency of its photons: radio waves, microwaves, infrared light, visible light, ultraviolet light, X-rays, and gamma rays. Visible light can be further separated into the colors red, orange, yellow, green, blue, and purple based on its frequency. Photons can be generated in a variety of ways, each involving the motion of charged particles. They can be emitted via blackbody radiation (an old-fashioned light bulb), spontaneous emission (neon lights), or even radioactive decay (nuclear waste).
To unlock this lesson you must be a Study.com Member.
Create your account
Video Transcript
Definition of a Photon
A photon is the quantum of electromagnetic radiation. The term quantum is the smallest elemental unit of a quantity, or the smallest discrete amount of something. Thus, one quantum of electromagnetic energy is called a photon. The plural of quantum is quanta.
The concept of photons and quanta comes from quantum mechanics and quantum theory. Quantum mechanics is a mathematical model that describes the behavior of particles on an atomic and subatomic scale. It demonstrates that matter and energy are quantized, or come in small discrete bundles, on the smallest scales imaginable. A photon propagates at the speed of light.
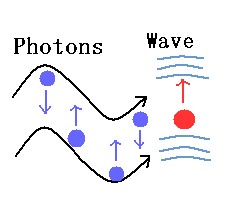
A photon describes the particle properties of an electromagnetic wave instead of the overall wave itself. In other words, we can picture an electromagnetic wave as being made up of individual particles called photons. Both representations are correct and reciprocal views of electromagnetic waves. For example, light exhibits wave properties under conditions of refraction or interference. Refraction is when light changes direction as it goes from one medium to another (i.e. from air to water), and interference is when light waves interfere with other light waves.Particle properties are exhibited under conditions of emission or absorption of light.
Energy
The idea of quantum mechanics and photons originated from scientists' observations of the photoelectric effect. The photoelectric effect is where light striking a surface causes electrons to be ejected from the metal. Scientists were unable to explain this phenomenon, but eventually the explanation came from quantum theory. Two common devices that use the photoelectric effect or similar process are laser printers and digital cameras.
What they found was that the energy in each quantum of light depends on the frequency of the light. In particular, the energy of a photon equals Planck's constant times the frequency of the radiation. Mathematically, this is given by the equation E = hf. Planck's constant is the fundamental constant of quantum theory that determines the scale of the small-scale world. Planck's constant = 6.63 * 10-34 joule-second (J-s). The total energy in an electromagnetic wave is the sum of the energies of each photon in the wave.
The energy of a photon is so small that we usually measure it in electronvolts (eV). One eV is the potential energy of each electron in a 1-volt battery. One eV is equal to 1.6 * 10-19 joules (J). Therefore, we need to convert Planck's constant to appropriate units, which are electronvolts/hertz (eV/Hz). In eV/Hz, Planck's constant is 4.136 * 10-15 eV/Hz.
Wavelength
The wavelength of a photon is the same as the wavelength of the electromagnetic wave of which it is a part. This picture shows the wavelength of the electromagnetic spectrum.

The smaller the wavelength, the more photon energy. Thus, a photon of an x-ray has much more energy than that of visible light. This is why too many x-rays are harmful to the human body. Note that since electromagnetic waves travel at the speed of light, frequency and wavelength for electromagnetic waves are related by wavelength = speed of light/frequency.
Calculation of Photon Energy
We will now explore an example of calculating photon energy using red light. The frequency of red light is 4.3 * 1014 Hz. Therefore, we have E = (4.136 * 10-15 eV/Hz)(4.3 * 1014 Hz) = 1.78 eV. We follow similar procedures for calculating photon energy by substituting in the appropriate frequency of radiation of the electromagnetic wave.
Lesson Summary
A photon is the quantum of electromagnetic radiation that describes the particle properties of an electromagnetic wave. The energy of a photon is given by the equation E = hf, where E is energy, h is Planck's constant, and f is frequency. This equation tells us that as the frequency of radiation increases (wavelength decreases), the photon energy increases. The wavelength of a photon depends on the frequency of radiation of the electromagnetic wave and is given by the relationship wavelength = speed of light/frequency.
Learning Outcomes
This lesson should teach you to:
- Define photon, quantum, Planck's constant, and electronvolt
- Recall the equation for the energy of a photon
- Calculate the energy of a photon
- Recall the relationship between frequency and wavelength for electromagnetic waves
To unlock this lesson you must be a Study.com Member.
Create your account